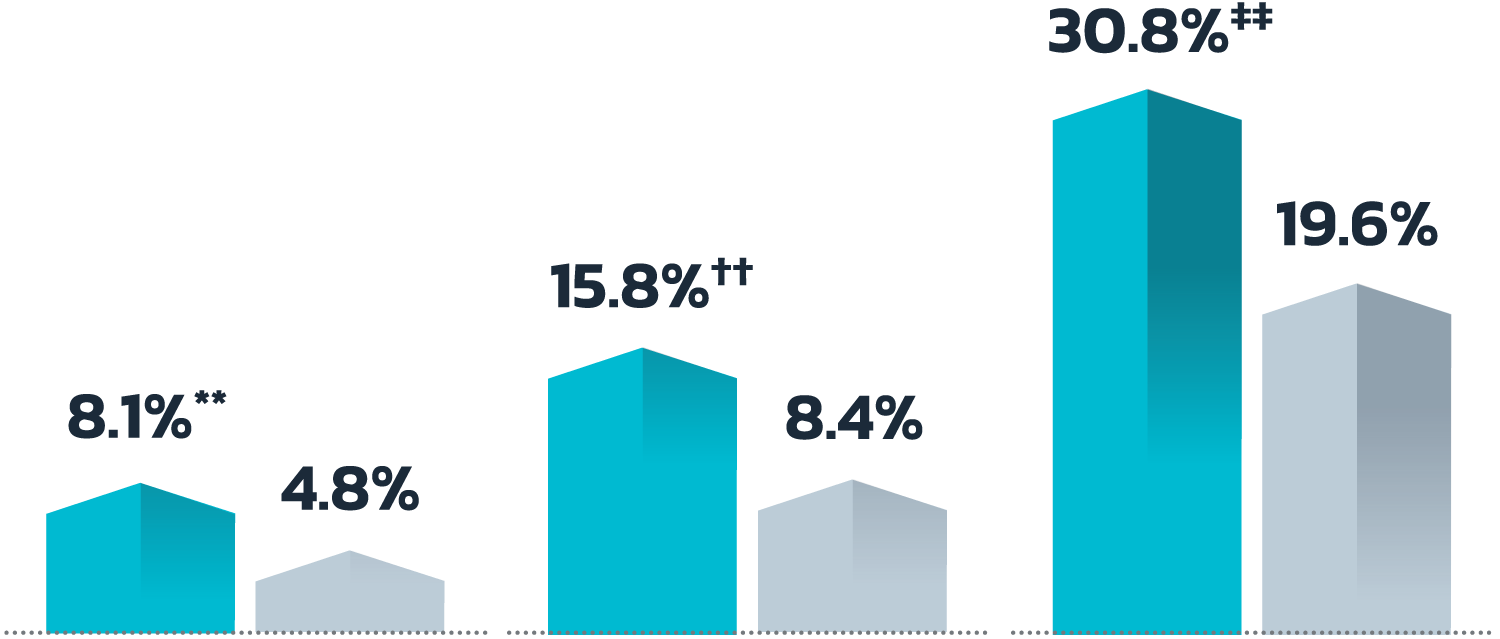
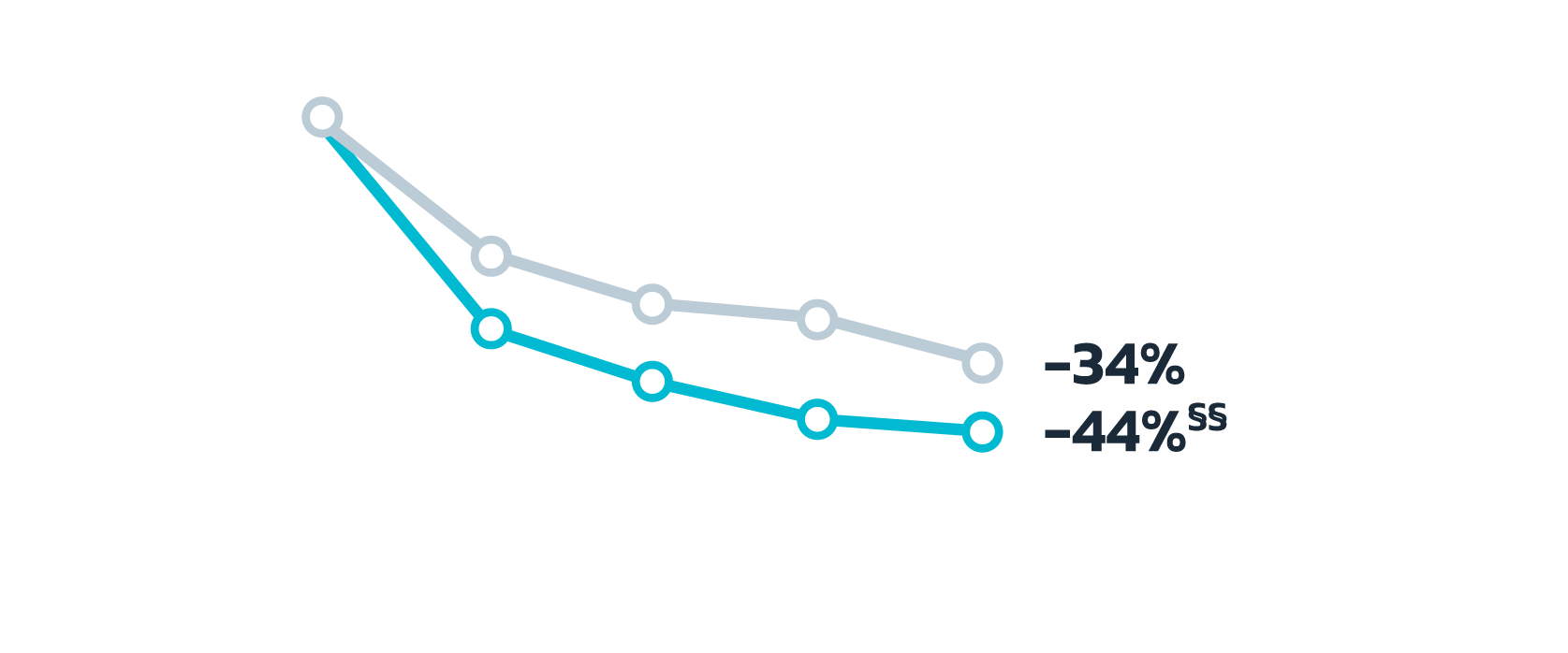
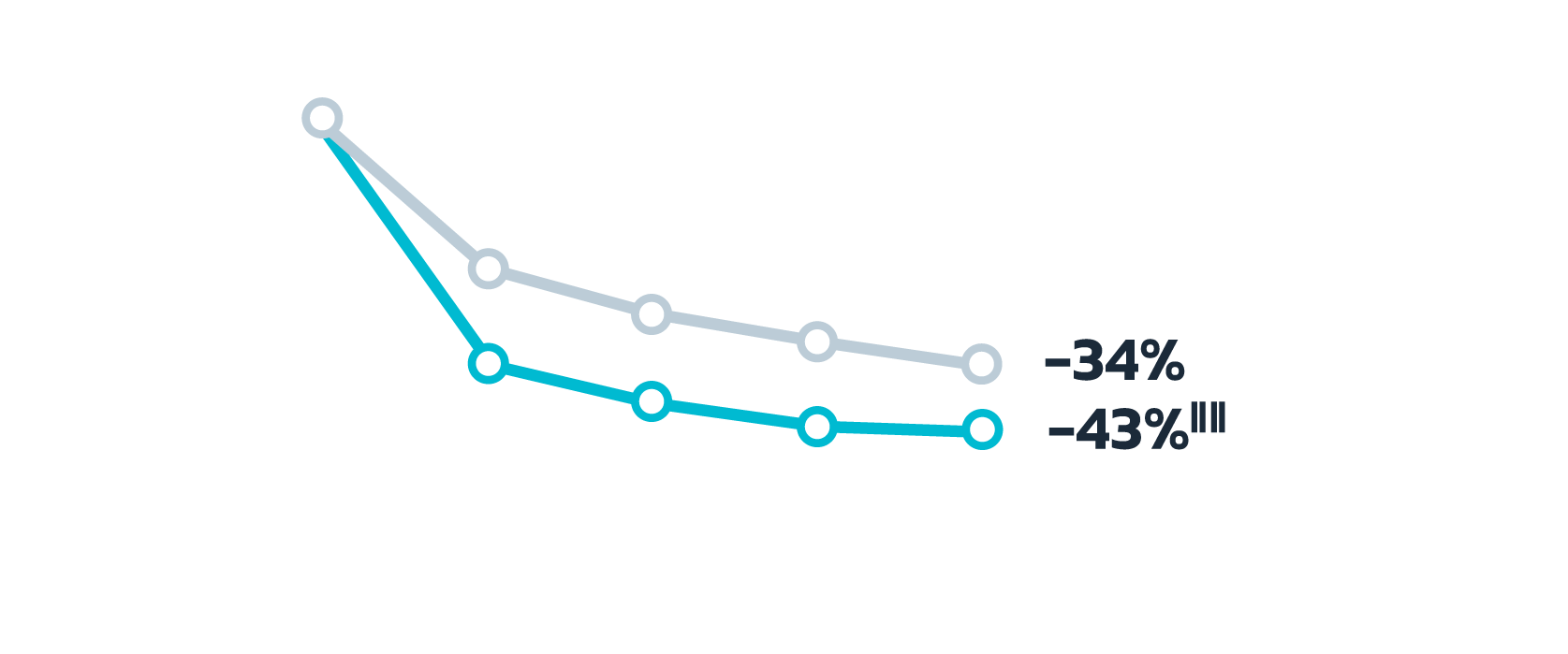
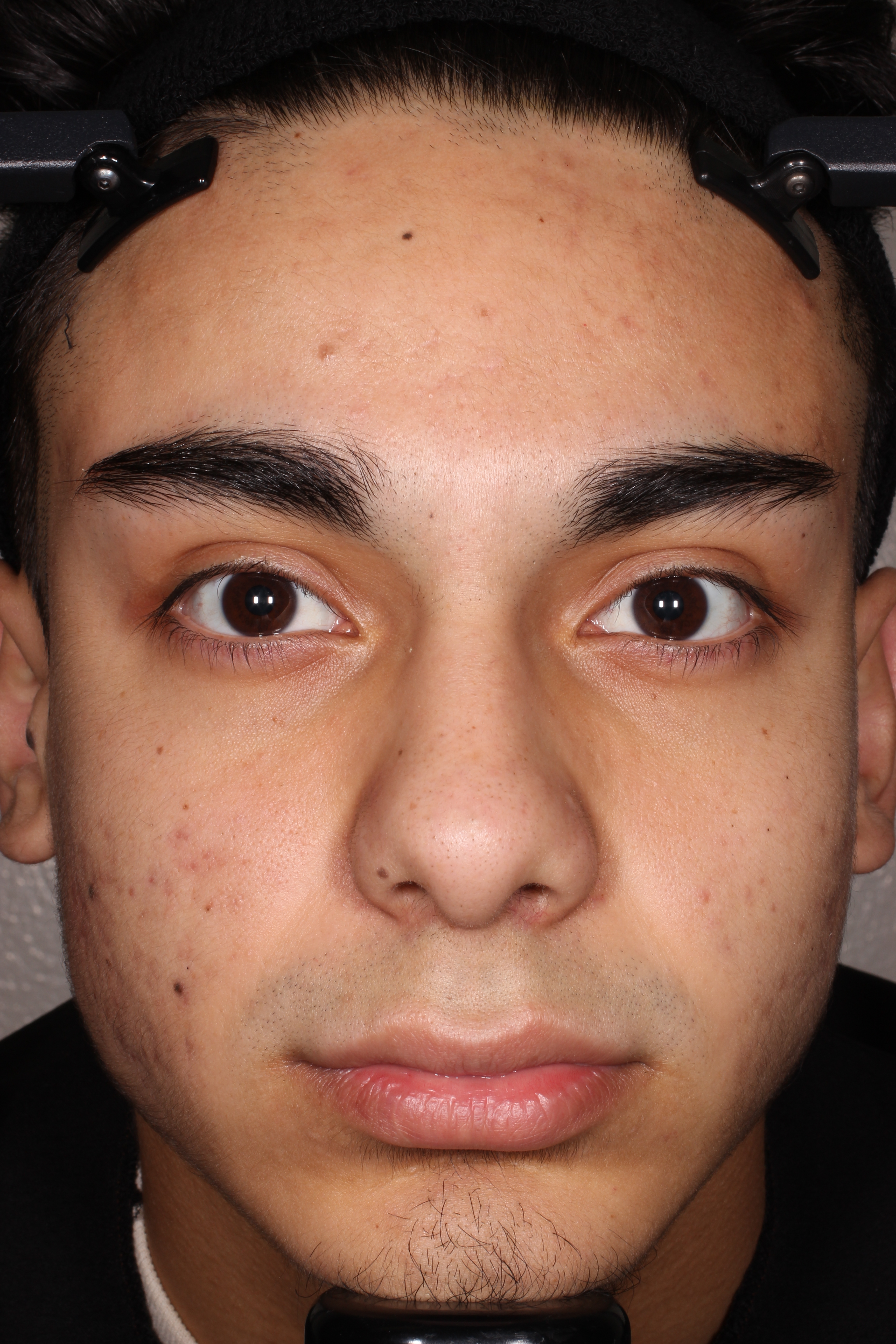
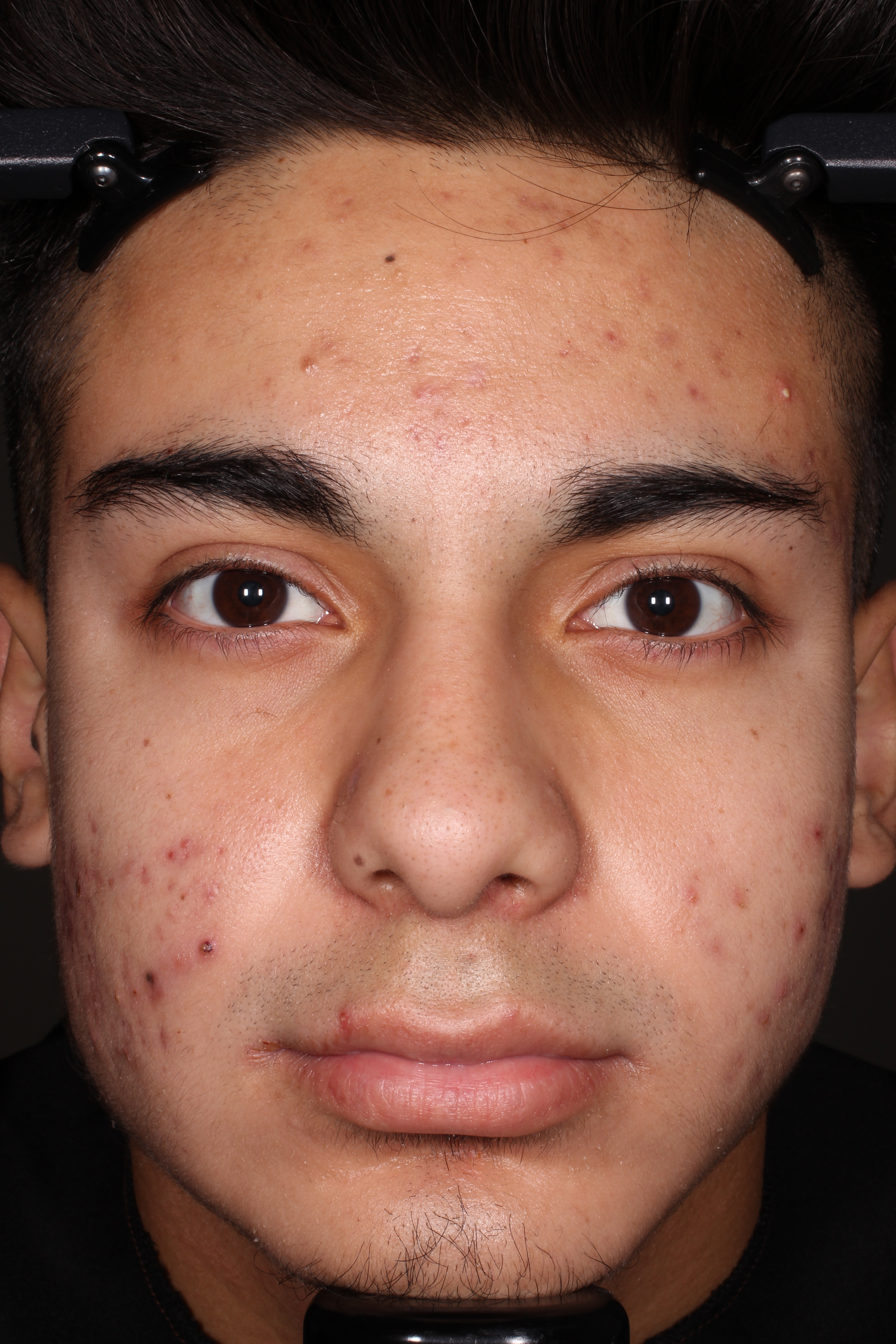
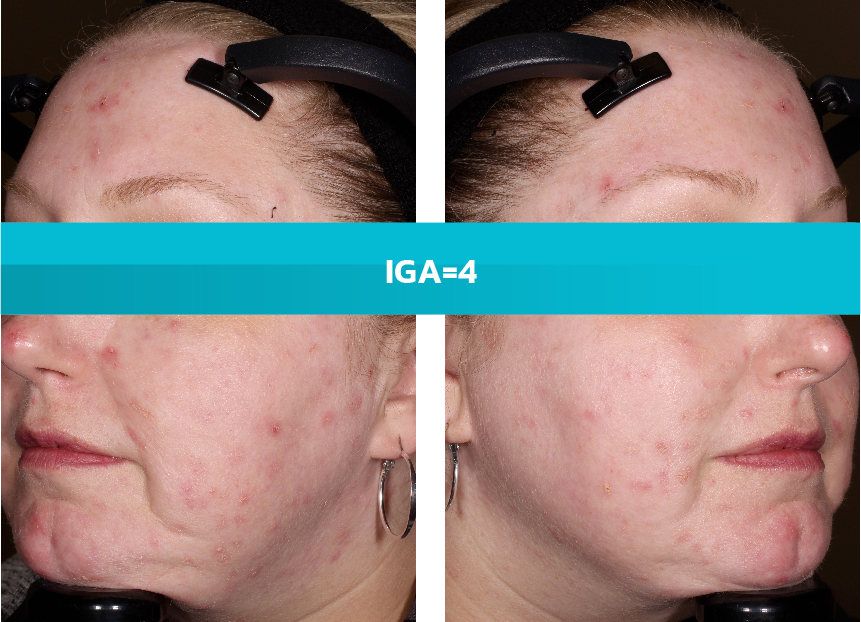
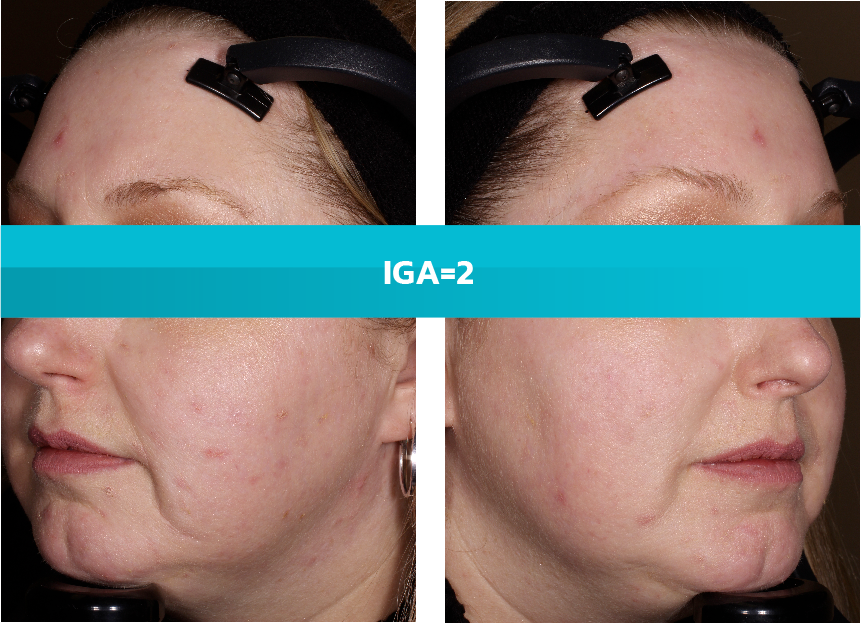
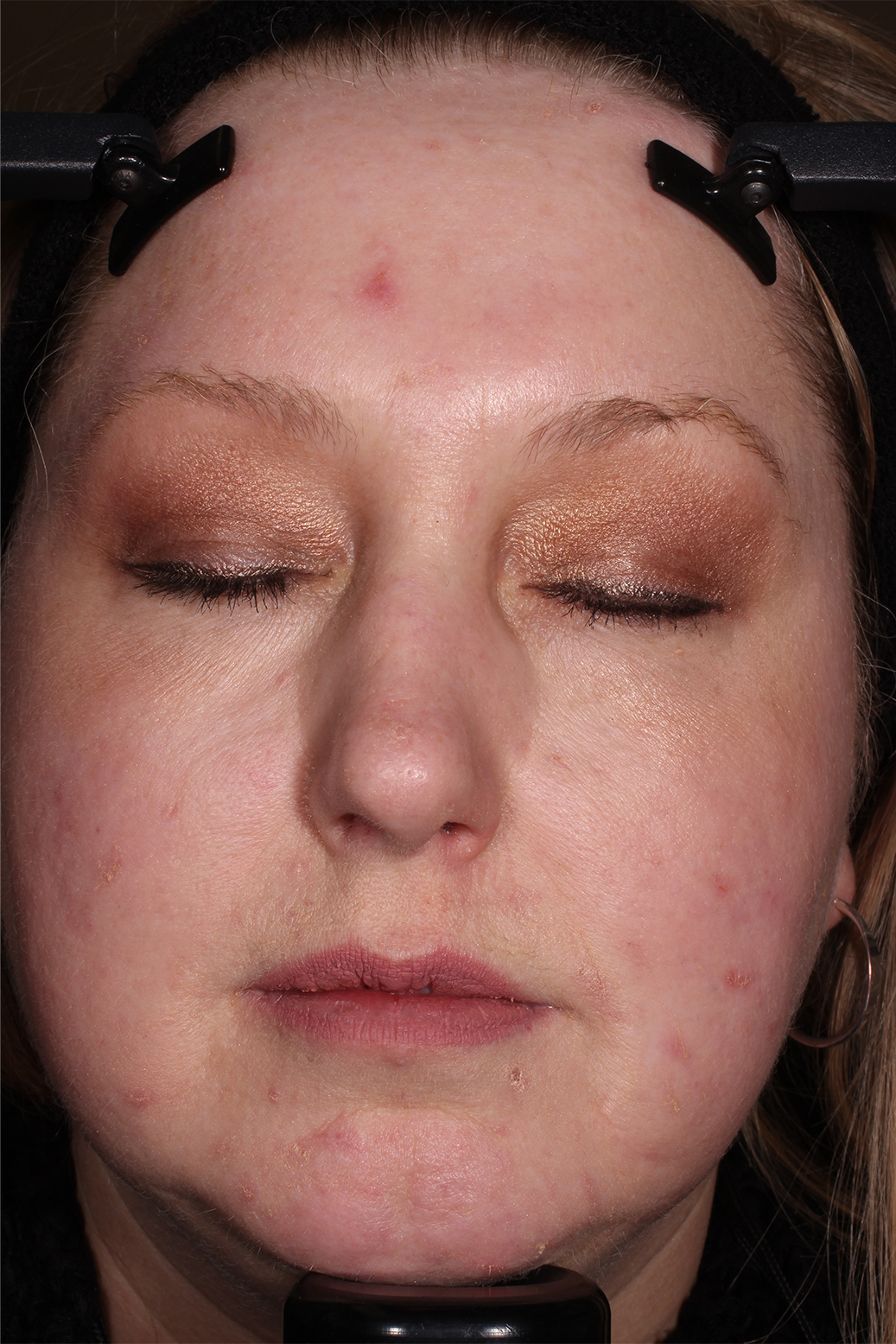
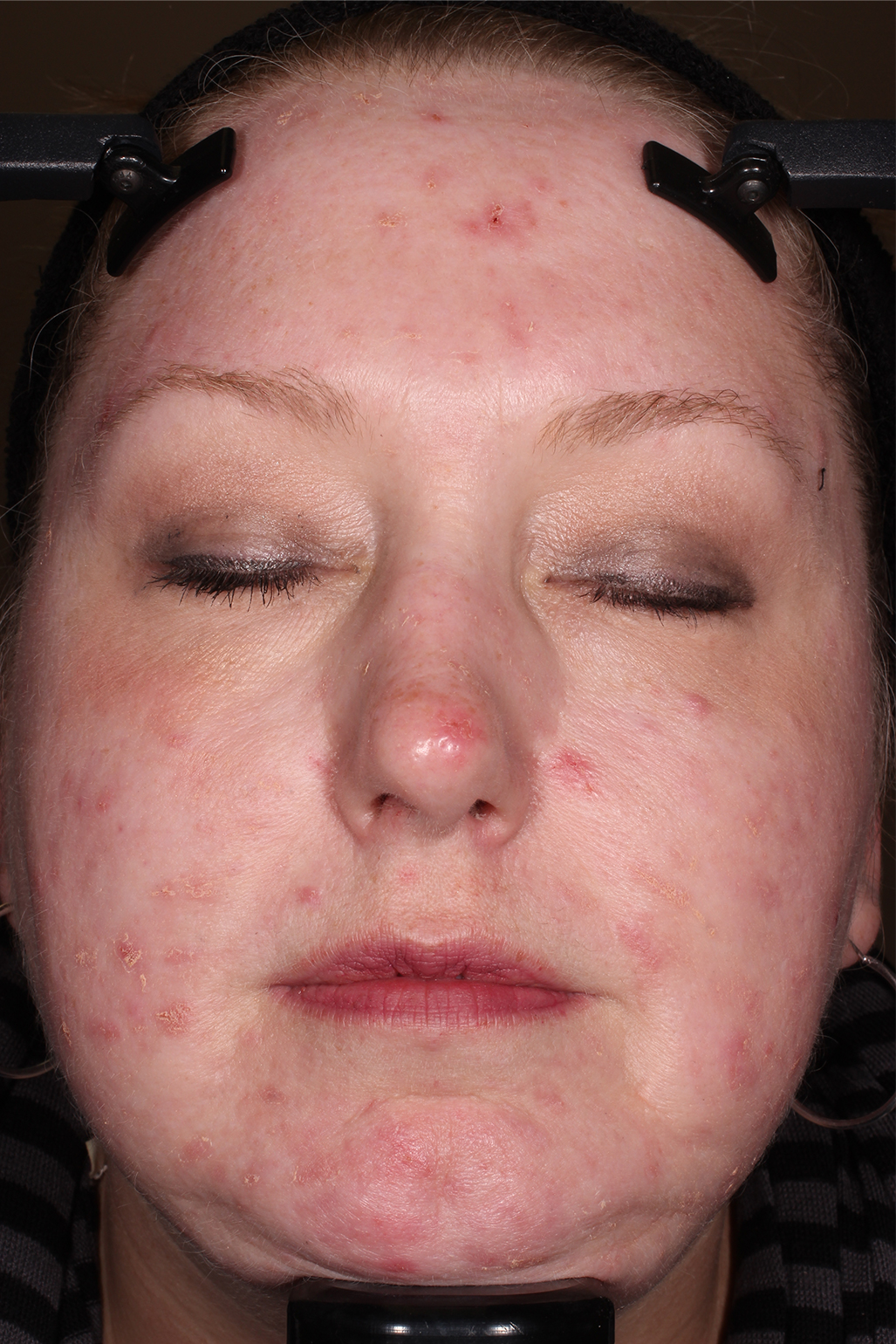
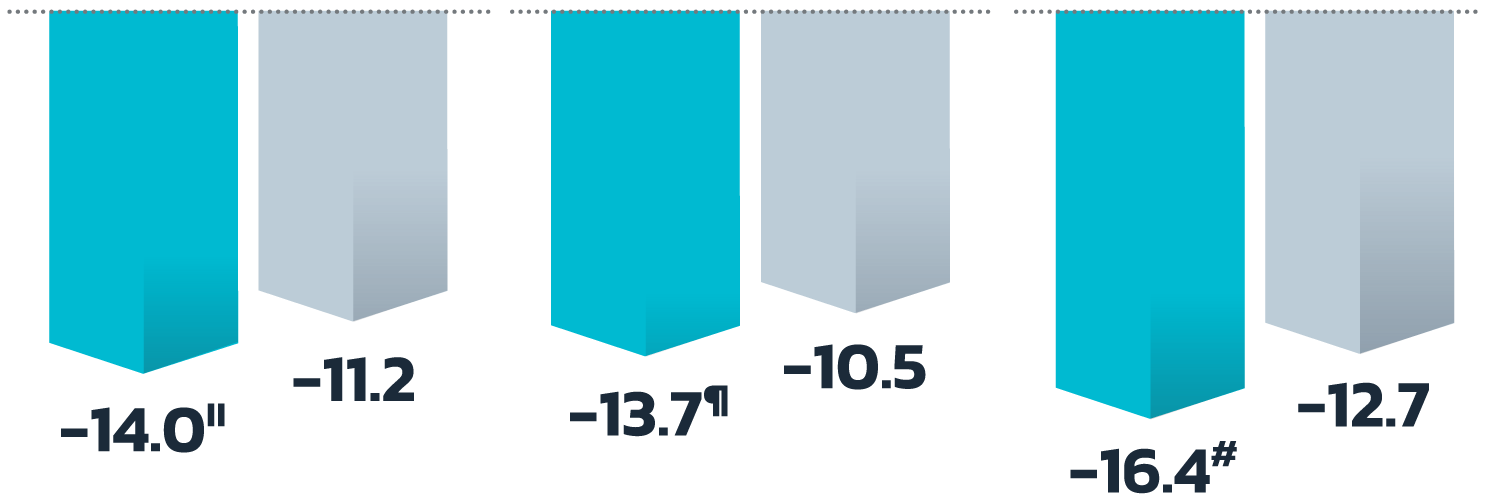- Adult patients, 18 years or older with acne vulgaris, were administered 1 single dose of oral minocycline extended-release tablets dosed 1 mg/kg
- Following a 10-day wash-out period, patients then began topical Amzeeq 4% therapy dosed 4 g/day (8x the recommended dose in clinical trials) applied for 21 days†
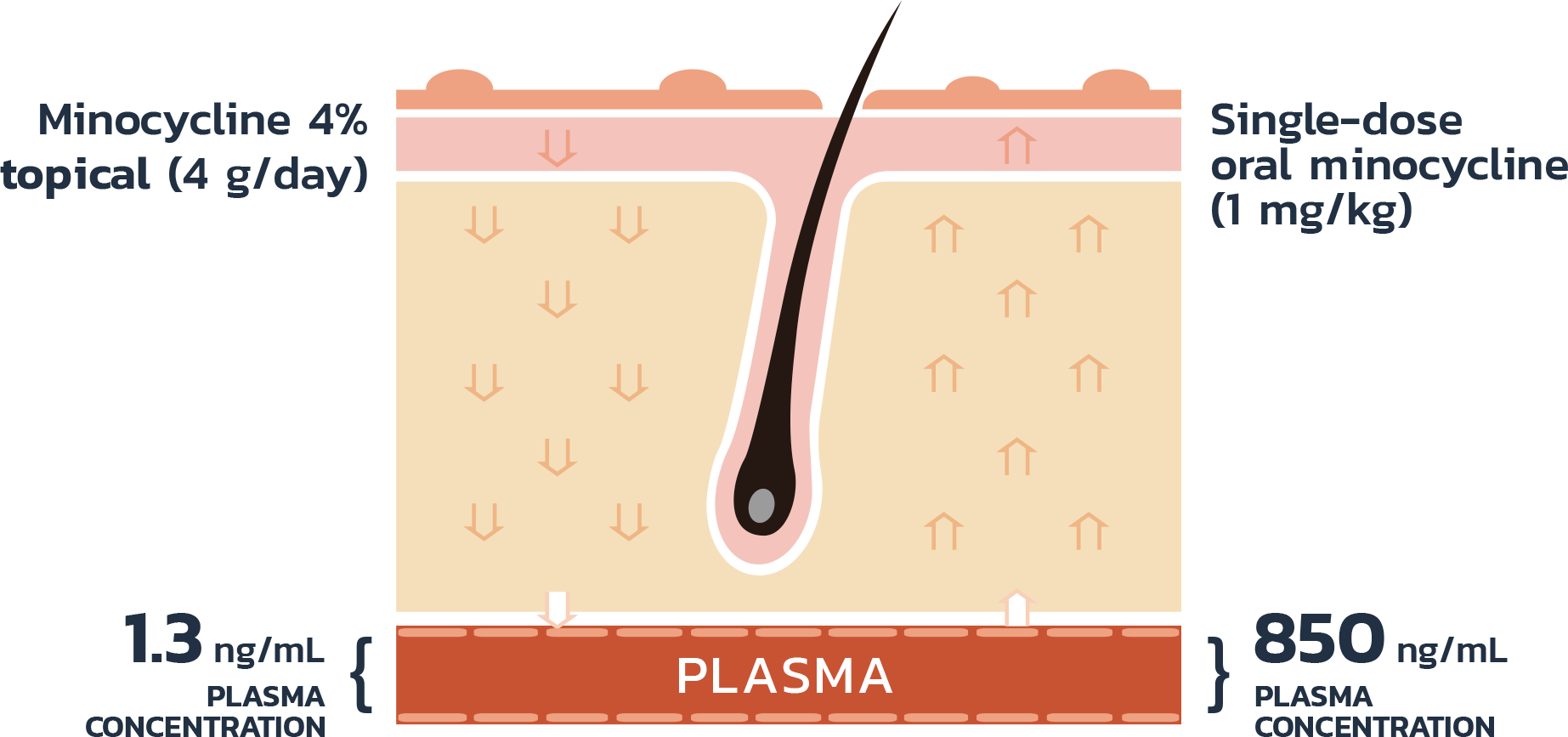
- The overall pediatric population showed 2.4-fold and 2.7-fold higher Cmax and AUCO-24h compared to the adult population
-
There were no significant differences in the minocycline
pharmacokinetics within the pediatric population among
subjects
10 years to less than 17 years of age with acne vulgaris
- *730-765.
- †Blood samples were obtained before and after administration of oral minocycline and each topical application of Amzeeq 4% on days 1, 12, and 21.